IONIC is composed of metals and nonmetals while COVALENT is composed of two nonmetal elements.
If it is a covalent bond, we can classify it as POLAR or NON-POLAR.
POLAR - Unequal sharing of electrons
NON-POLAR - Equal sharing of electrons
One way to determine if it is polar or non-polar is the electronegativity.
We have to subtract the electronegativity and if the result is:
0 - Non-polar
0.1- 1.6 - Polar
If it is 1.7 up, it is Ionic.
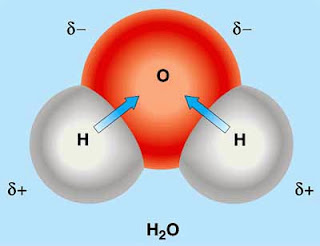
Dipole - Partial positive and negative charge in same direction.
If we cancel, this is non-polar. Example is CO2
If it is i the same direction, it is polar. Example is H2O
And also, all symmetrical shape and hydrocarbons are non polar. Just so you know. ;)

No comments:
Post a Comment